Learn More: Swinging Bucket vs. Fixed Angle Rotors
Swinging Bucket vs. Fixed Angle Rotors
 Researchers in biochemistry, molecular biology, medicine and other fields commonly use centrifugation to separate sample components, a technology that relies on gravity and mass: particles in a heterogeneous solution will over time separate based on size and density. Smaller, less-dense particles may also migrate downward, but at a much slower rate; some particles will never settle, but remain suspended in solution. Because centrifuges accelerate and enhance this process, they have found widespread use across the sciences since the late 19th century.
Researchers in biochemistry, molecular biology, medicine and other fields commonly use centrifugation to separate sample components, a technology that relies on gravity and mass: particles in a heterogeneous solution will over time separate based on size and density. Smaller, less-dense particles may also migrate downward, but at a much slower rate; some particles will never settle, but remain suspended in solution. Because centrifuges accelerate and enhance this process, they have found widespread use across the sciences since the late 19th century.
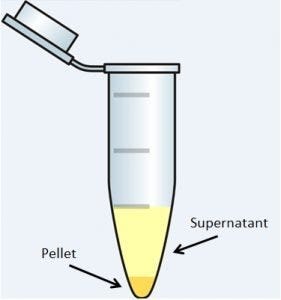 Centrifugation utilizes centripetal force to drive the separation of a heterogeneous solution into supernatant and pellet. The modern centrifuge amplifies the force of gravity by spinning solutions at a high rate of speed to increase the rate of sedimentation. During this process, materials with a high particle density will sediment towards the axis of centrifugation (down the tube), while materials with a lower particle density will sediment away from the axis of centrifugation. For example, bacterial cells in suspension have a high particle density and will tend to settle to the bottom.
Centrifugation utilizes centripetal force to drive the separation of a heterogeneous solution into supernatant and pellet. The modern centrifuge amplifies the force of gravity by spinning solutions at a high rate of speed to increase the rate of sedimentation. During this process, materials with a high particle density will sediment towards the axis of centrifugation (down the tube), while materials with a lower particle density will sediment away from the axis of centrifugation. For example, bacterial cells in suspension have a high particle density and will tend to settle to the bottom.
A process that normally takes hours or days under the force of normal gravity (1 g) can be shortened to minutes when a force of 10,000 g is applied with a relatively inexpensive and easy-to-use piece of equipment. The faster the centrifuge turns (i.e., the higher the RPM), the faster the faster the sedimentation. Relative centrifugal force (RCF) is another way of expressing the gravitational force (g-force), or the turning of the rotor, which allows researchers to easily separate tissues, cells, organelles, and macromolecules for further investigation.
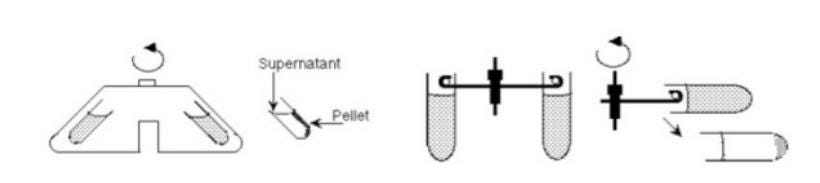
 The centrifuge incorporates one of two types of rotors, which house the tubes where separation occurs. Fixed-angle rotors hold tubes at a stable angle (typically 45°) relative to the axis of rotation. A swinging-bucket rotor swings out when centripetal force is applied and holds the cells at an approximate 90° angle relative to the angle of rotation. Recall that more dense materials will separate towards the angle of the centripetal force. Therefore in swinging-bucket rotors, the pelleted material will form at the bottom of the conical centrifuge tube whereas tubes processed in a fixed-angle rotor will form sedimentation on the side. This could be problematic if the solids get caught in the angle of the tube.
The centrifuge incorporates one of two types of rotors, which house the tubes where separation occurs. Fixed-angle rotors hold tubes at a stable angle (typically 45°) relative to the axis of rotation. A swinging-bucket rotor swings out when centripetal force is applied and holds the cells at an approximate 90° angle relative to the angle of rotation. Recall that more dense materials will separate towards the angle of the centripetal force. Therefore in swinging-bucket rotors, the pelleted material will form at the bottom of the conical centrifuge tube whereas tubes processed in a fixed-angle rotor will form sedimentation on the side. This could be problematic if the solids get caught in the angle of the tube.
 The fact that swinging-bucket rotors offer a superior sedimentation location makes them the obvious choice for centrifuging samples under most conditions; however, fixed-angle rotors offer valuable benefits that often make them a more desirable choice. What are they? First, due to its simple and efficient tube spacing, a fixed-angle rotor can hold a greater quantity of tubes compared to its swinging-bucket counterpart, making it more practical for high-throughput applications. Next, as a result of the rigid design of the metal alloy material, fixed rotors can withstand much higher gravitational forces necessary when separating biological macromolecules such as RNA, DNA and protein. Finally, researchers often wish to wash the pelleted material repeatedly after centrifugation. The placement of the pellet on the side of the tube allows it to be washed with solution more easily without the possibility of disturbing the pellet.
The fact that swinging-bucket rotors offer a superior sedimentation location makes them the obvious choice for centrifuging samples under most conditions; however, fixed-angle rotors offer valuable benefits that often make them a more desirable choice. What are they? First, due to its simple and efficient tube spacing, a fixed-angle rotor can hold a greater quantity of tubes compared to its swinging-bucket counterpart, making it more practical for high-throughput applications. Next, as a result of the rigid design of the metal alloy material, fixed rotors can withstand much higher gravitational forces necessary when separating biological macromolecules such as RNA, DNA and protein. Finally, researchers often wish to wash the pelleted material repeatedly after centrifugation. The placement of the pellet on the side of the tube allows it to be washed with solution more easily without the possibility of disturbing the pellet.



