PCR Thermal Cyclers Comparison Chart Overview
What is a PCR Thermal Cycler?
PCR thermal cyclers optimize sample conditions to empower the amplification of target nucleic acid sequences for quantitative and qualitative analysis. Thermal cyclers are designed to perform two types of polymerase chain reaction (PCR) methods: end-point, or standard PCR, and real-time qPCR.
Standard PCR thermal cyclers amplify the target DNA or RNA sequence but do not include the software (or detector) to measure the rate of amplification in real-time.
Real-Time qPCR vs Standard PCR Thermal Cyclers
What is the difference between a real-time PCR system and a standard PCR system?
Real-time PCR systems quantity the copies of target DNA or RNA produced as the PCR reaction proceeds, establishing a Ct value (or threshold cycle) correlating to the point in the reaction when amplification begins to exponentially increase. As such, real-time PCR systems provide both a qualitative and quantitative examination of the sample, whereas standard PCR systems provide only a qualitative analysis of the sample.
Standard PCR systems include a sample block (compatible with microplates, microtubes, or PCR strips), heating and cooling elements, and an onboard controller. The heat is transferred between the element and the sample block (containing the specimens) through the Peltier effect, ensuring fast ramping and cooling rates and tight temperature tolerances.
Real-time PCR system features also include a sample block, heating/cooling elements and a controller, as well as a light source (often a Tungsten lamp), light filter, and light detector. The real-time PCR thermal cycler’s on-board software includes additional functionality to chart the results of the reactions as they occur, assigning amplification curves as Ct values to each positive specimen.
Polymerase Chain Reactions - Standard vs Real-Time PCR Reactions
Prior to loading the samples into the thermal cycler, PCR reactions must contain several reagents (or Master Mixes) to ensure a successful amplification of the target sequence. For standard PCR reactions, the samples must include the target nucleic acid, nuclease-free (PCR-grade) water, dNTPs (di-nucleotide tri-phosphates), DNA polymerase, and custom oligonucleotides designed to bind upstream and downstream of the target gene sequence. For real-time PCR reactions, the samples must include each of the above reagents, as well as a fluorescent probe, or reporter molecule, designed to anneal to the target sequence.
Standard PCR Protocol Steps Defined
What is the standard protocol for polymerase chain reaction (PCR) techniques?
The standard PCR protocol consists of an initial step followed by a precise, 2-step temperature sequence, repeated for 40 cycles. The first step, or denaturation step, involves heating the sample to 95°C for 3 minutes, to separate, or denature, the double-stranded target DNA into single strands. The second step, called the annealing step, consists of cooling the sample to 60°C, the ideal temperature for the primers and DNA polymerase to bind, or anneal, to the target sequence. The third step, or the extension step, involves heating the sample to 72°C, to allow the DNA polymerase to form a new complementary strand of DNA, using the dNTPs (or di-nucleotide tri-phosphates). The annealing and extension steps are repeated for 39 additional cycles to form millions of copies of the target DNA sequence.
Real-Time PCR Protocol Defined
The real-time PCR protocol mirrors the standard PCR protocol with one exception: the annealing step also involves the binding of the fluorescent probe, or reporter molecule, to the target DNA strand. As the DNA polymerase forms a new complementary strand during the extension step, the probe emits light at a specified wavelength. The light is measured by the detector as the reaction proceeds through the 40-cycle protocol, giving the researcher an on-demand analysis of the amplification rate within each sample. With some variance due to protocol differences, normal PCR runs last between 90 and 100 minutes.
On-Board Software for PCR Thermal Cyclers
The on-board software installed on a standard PCR thermal cycler, such as Benchmark’s TC Series, includes an output of the reaction protocol, a status indicator defining the current step of the reaction, and an estimated completion time. More advanced standard thermal cyclers include user-defined protocols, password protections, and temperature optimization wizards.
Analytik Jena’s QTOWER 3 - PCR Amplification Curve
Real-time PCR thermal cyclers, such as Analytik Jena’s QTOWER 3, feature more sophisticated software that displays an on-demand output of reaction results, called a PCR amplification curve, and a measurement of the Ct value, or cycle during which the sample target sequence begins to amplify exponentially. If the target sequence does not exponentially amplify until the reaction is nearing completion (such as cycle 35 or higher), the researcher can reasonably infer that the sample contained fewer copies of the target sequence. If the target sequence begins to exponentially amplify at an earlier cycle (such as 25), the researcher can infer that the sample contained more copies of the target sequence.
Standard vs Real-Time PCR Applications
Standard and real-time PCR are used in a wide variety of applications, including forensics, environmental science, clinical diagnostics (such as COVID testing), cloning, genotyping, mutation detection (for cancer screening), and paternity testing.
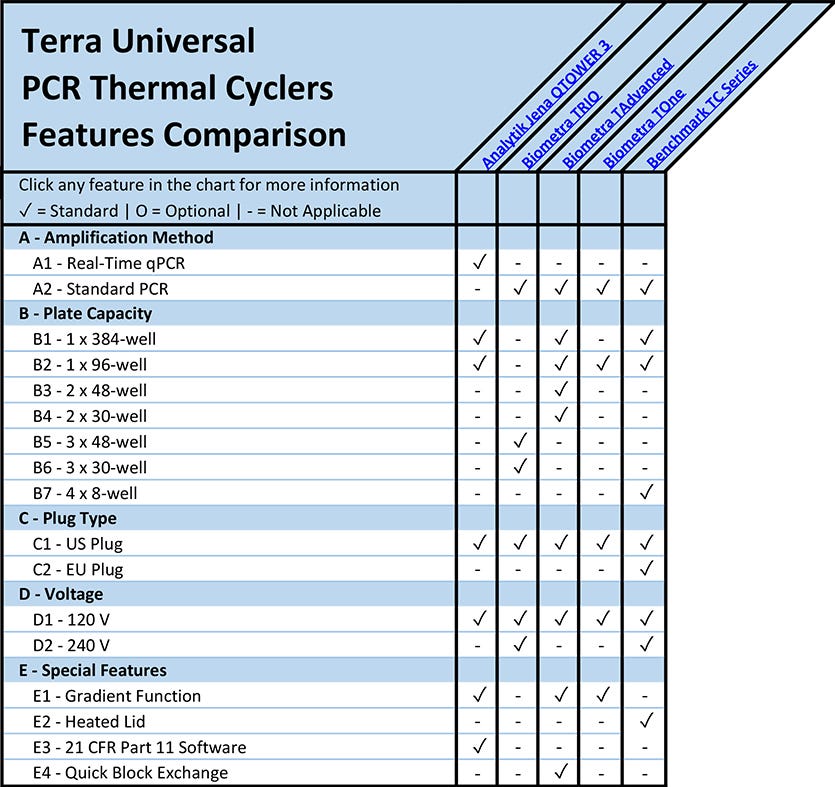
A - Amplification Method
(back to chart)
A1 - Real-Time qPCR
In real-time quantitative PCR, the target sequence, or amplicon, is measured as the reaction progresses toward completion, with copy number quantitation after each cycle.
What is the advantage of real-time PCR?
Real-time PCR does not require additional, lengthy end-point detection steps, such as gel electrophoresis, but provides the researcher with a quantitative and qualitative sample analysis in fewer steps than conventional PCR. The process is more costly than standard PCR, but the protocol is less laborious, provides on-demand analysis (for time-sensitive research), and is less prone to cross-contamination.
A2 - Standard End-Point PCR
In standard PCR, or conventional end-point PCR, the DNA target sequence, or amplicon, is detected using end-point analysis, normally accomplished by gel electrophoresis. Conventional PCR provides a qualitative analysis of the sample, but not a quantitative assessment. The process is less costly than real-time PCR, but the protocol is longer, more laborious, and more vulnerable to sample cross-contamination.
B - PCR Thermal Cyclers Plate Capacity
(back to chart)
PCR thermal cyclers include plate blocks compatible with differing quantities of microtubes, PCR strips and microplates. Biometra’s TAdvance thermal cycler features a quick-exchange, interchangeable plate block compatible with 96-well and 384-well microplates, 0.5 ml microtubes, and 0.2 ml PCR strips. Biometra’s TRIO PCR thermal cycler features three distinct plate blocks, allowing users the flexibility to run three separate PCR protocols in parallel. Each plate block on the Biometra TRIO accommodates up to 48 samples.
C - Thermal Cycler Wall Plug Type
(back to chart)
U.S. thermal cycler power cords are compatible with standard 120-volt and 240-volt outlets used throughout North America. E.U. plugs are compatible with 240 volt, 50-hertz outlets used through mainland Europe. Plug adapters are available for Schuko, U.K. and Australian outlets.
D - Thermal Cycler Voltage
(back to chart)
120-volt connections are suitable for standard laboratory power outlets in the United States. 240-volt connections, common in Mainland Europe, require less current (amperage) and smaller conductors than equipment designed to operate at 120-volt. Specialty high-voltage equipment requires 480-volt connections and carries additional protections against electrical hazards.
E - Special Thermal Cycler Features
(back to chart)
E1 - Thermal Cycler Gradient Function for PCR
Gradient PCR is a technique that allows the user to determine the optimal annealing temperature for the primer, and possibly, probe mix within the chosen assay. Gradient thermal cyclers, like Biometra’s TOne, include a single sample block with a heating element installed on one side of the block and a cooling element installed on the opposite side. The heating and cooling elements work in tandem to generate a gradient across the sample block, allowing for positive control samples to amplify under differing annealing conditions. End-point analysis helps to determine which annealing temperature supported the most robust amplification.
E2 - Thermal Cyclers with Heated Lid
Benchmark’s TC Series thermal cyclers include a heated lid to minimize sample evaporation by reducing condensation. Heated lids also help ensure that the sample temperature remains homogenous throughout the PCR run.
E3 - 21 CFR Part 11 Thermal Cycler Software
Analytik Jena’s QTOWER 3 PCR thermal cyclers feature software compliant with the FDA’s 21 CFR Part 11 requirements. The software includes individual user authentication, lock-out access after a set period of inactivity, a comprehensive event log, and e-signature functionality.
E4 - Quick Block Thermal Cycler Exchange
Biometra’s TAdvance thermal cycler features a quick-exchange, interchangeable plate block compatible with 96-well and 384-well microplates, 0.5 ml microtubes, and 0.2 ml PCR strips.
Where Can I Buy PCR Thermal Cyclers Online?
Laboratory-Equipment.com is a specialty division of Terra Universal. For nearly 40 years, Terra Universal has served the life science, pharmaceutical, biotechnology, and medical device markets. Customers appreciate a worldwide network of reps, factory-direct support, and ready-to-ship items available from Terra's on-shore manufacturing and warehouse facilities in Fullerton, California.
Shop lab thermal cyclers online for a variety of pharmaceutical, laboratory, life science research, and analytical environments. Contact a Laboratory-equipment.com specialist through web chat, email, or phone for pricing or a same-day quote.
Laboratory-Equipment.com | U.S. Customer Service
Email: Info@Laboratory-Equipment.com
Phone: (714) 578-6016
