Water’s ability to dissolve compounds, along with its polarity, bonding, melting, boiling and freezing points, heat absorption, and vaporization characteristics arguably make it the most versatile substance we know. It’s also ubiquitous and plentiful: the earth can’t live without it, most plants and animals can’t exist without it, and scientists can’t operate labs without it.
Water is the most common reagent used in the laboratory, and while water quality can often be overlooked, the grade of water being used in an application is critical. Minute traces of salts or biological contaminants can result in unfortunate consequences when culturing cells or performing analytical measurements of biological macromolecules.

Water, water everywhere, but is any of it good enough for sample analysis?
With that being said, how is water quality measured and how do researchers determine the purity of water necessary for their process? Water quality is measured by examining a set of parameters from a water sample and comparing that to set values to achieve a water purity rating. These ratings were established by the international rating organization ASTM and ranked from Type 1 (the most pure) to Type 4 (the least pure). Let’s look at specifications that scientists measure to determine a purity rating:
Resistivity: Resistivity is a measurement of water’s ability to resist or conduct an electrical charge. Pure water has a resistance of 18.2 MΩ × cm at 25°C. The resistance of water can be easily and inexpensively monitored in a laboratory. As the level of ionic impurities (salts from minerals in the earth’s crust) increases in water, the resistivity decreases. Resistivity will only measure ionic impurities and does not account for organic compounds from biological contaminants.
Total Organic Carbon (TOC): TOC is a measurement of the amount of organic carbon that is found in a water sample. The measurement is calculated from the total carbon in a water sample, subtracting out inorganic carbon (from dissolved carbon dioxide and carbonic acid salts) to arrive at the TOC Count.
The TOC level can be affected by a host of contaminants in a water sample, such as decomposing biological material, bacterial growth or the chemical activity (metabolism) of living organisms. High TOC measurements can be indicative of bacterial biofilm growth within a water supply or other upstream biological contamination. TOC is typically measured in parts per billion (ppb), with Type 1 water systems having less than 10 ppb.
Bacteria: Bacteria are a common source of water contamination and often included in the measurement of laboratory water quality. The number of bacterial cells with the potential to multiple and grow in the water is counted and reported as colony forming units (CFUs) per unit volume (ml). For Type 1 water systems, 1 ml of water must form fewer than 10 bacterial colonies.
Endotoxins: Endotoxins are lipopolysaccharide molecules released when bacterial cells die. Results of in vivo and in vitro experiments are negatively impacted when they are present, and labware is easily contaminated. Thus, measuring the amount of these toxins in a laboratory water system is significant when measuring water purity. Type 1 water quality systems typically have measurements of less than 0.03 Endotoxin Units per ml of water.
Level of Contaminants in Different Water Purity Types*
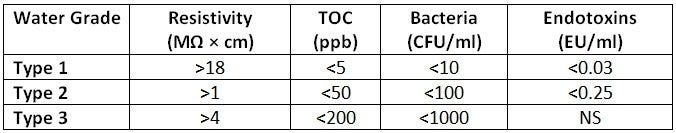
*According to ASTM Standards for Laboratory Reagent Water (ASTM D1193-91)
Water Quality Type by Applications
The National Institute of Health (NIH), Division of Technical Resources has released guidelines that detail the type of water purity system that should be used in various scientific disciplines and for specific applications. While Type 3 water is considered usable for autoclaving, glassware rinsing or washing and water baths, Type 1 and Type 2 water are indicated for most laboratory procedures. Type 2 water is generally suited for reagent and buffer mixtures in addition to cultured media preparation. Type 1 water is reserved for the most sensitive processes, such as High Performance Liquid Chromatography (HPLC) and trace analysis. The type of water required for a given application can be found summarized in this NIH table*:
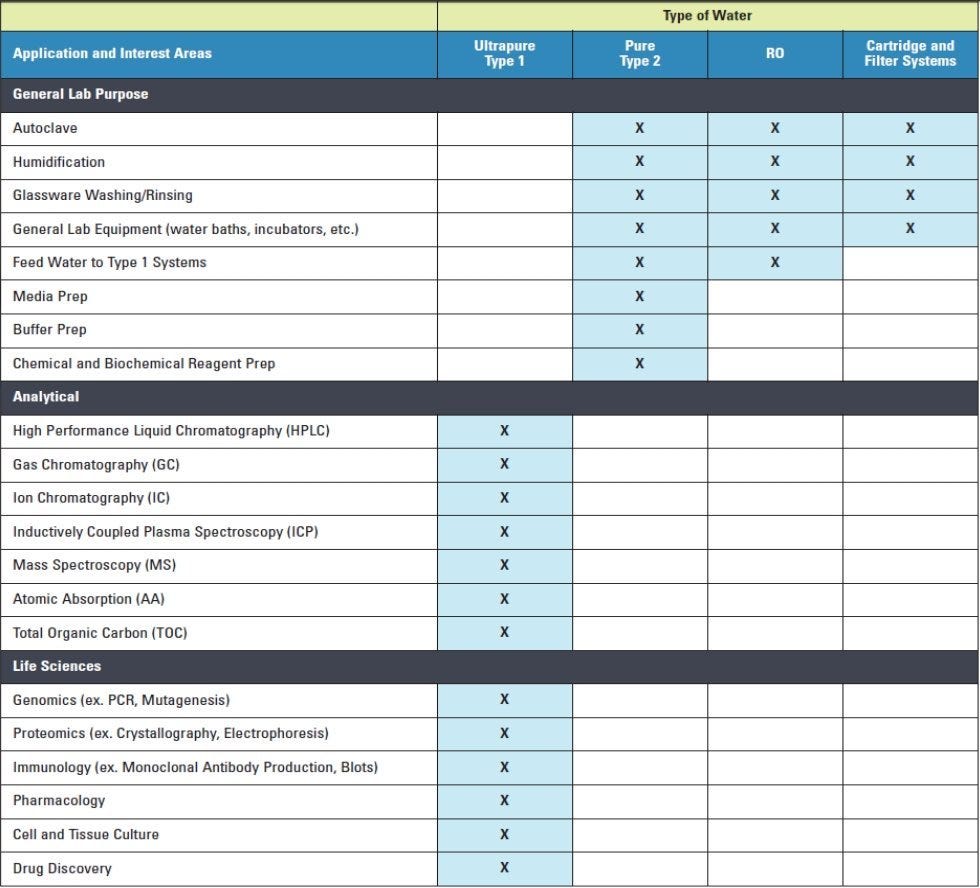
*RO is Reverse Osmosis, which can effectively remove bacteria and other contaminants, but not dissolved gasses.
Water is more complicated than simple hydrogen and oxygen. Like any chemical used in the laboratory, it has classifications and application-specific forms. Researchers should consider their analytical tasks and have the recommended water type on hand.
Learn more about water purification systems offered at Laboratory-Equipment.com.
Try before you buy!
DEMO MODELS AVAILABLE
Contact us for Demos, Samples, Brochures and more.
EMAILMon - Fri, 5:30am - 5:30pm PST