Electrophoresis is a common lab procedure for identifying and separating macromolecules. It was first observed in the early 1800s by a university scientist in Moscow. Like many discoveries, it was accidental, but has proven itself useful for many research scenarios. By applying electricity, technicians use the particles’ negative or positive charges to make them migrate through porous matrix, such as an agarose gel. When positively charged molecules are present in a sample, they will creep towards the negative current (cathode), while negatively charged molecules will migrate to the positive current (anode).
Besides a source of electricity and gel, this kinetic test requires buffer to help prevent temperature and pH extremes. The type of gel used depends on the sample and application. Gels are “solid,” but porous. Within the gel, larger molecules will travel more slowly and smaller molecules will move quickly. Therefore, molecular size is another way that the sample is separated and molecules identified.

Gel imaging system; works with a smart phone to capture photos. Photo: Accuris
So, now that we understand the kinetics of electrophoresis, how do we see where these particles are moving? The molecules may be “macro,” but they are still too small to observe with the naked eye. One last necessary ingredient for our experiment is stain. A group of dyes allow us to visualize the paths that the particles take. From there, we can capture images to document results.
DNA Gel Stains
Several choices exist for staining nucleic acids during gel electrophoresis. Ethidium Bromide (EtBr) is the most well-known and commonly used DNA dye. It is an intercalating agent that binds DNA and has a 20-fold increase in fluorescence when exposed to UV light. EtBr is the most inexpensive DNA stain, making it the ideal choice for many research laboratories. However, EtBr must be used with care, as it is a known mutagen. Additionally, EtBr requires exposure to UV light to be visualized, but molecular damage will occur. This presents a problem when the DNA is to be used for downstream applications such as cloning.
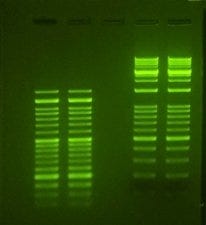
Nucleic acid stain, viewed through UV light. Photo: Accuris
Alternative DNA dyes, such as SYBR Safe, have been developed that do not share the same mutagenic properties as EtBR. SYBR Safe is not considered hazardous and can be disposed of using the lab’s regular sewage system. Additionally, it fluoresces under blue light, which prevents DNA damage that occurs when using UV light.
Many DNA stains have been shown to inhibit polymerase chain reaction (PCR). When high PCR efficiency is needed, such as real-time quantitative PCR, a viable DNA stain would be Eva Green. Compared to other common electrophoresis dyes, it restricts PCR to a lesser extent. Eva Green is also safer than traditional stains such as EtBr.
Protein Gel Stains
Similar to DNA gels, there are several options for staining protein gels. The two most common stains are Coomassie Brilliant Blue and Silver stain. Coomassie is an anionic dye, which binds nonspecifically to proteins. Protein bands are visualized as blue bands once the excess stain is removed. Coomassie staining is the most prevalent method for protein staining due to its ease-of-use and affordable cost.
Stain being added to samples prior to injection into electrophoresis gel. Photo: Accuris
Conversely, Silver stain is more sensitive than Coomassie for detecting proteins in relatively low quantity. Sensitivity comes with a price, though, as Silver stain can also bind to polysaccharides and nucleic acids, yielding spurious bands on a silver-stained gel.
Final Thoughts
Dye performance can also be affected by chemicals in electrophoresis gels and buffer solutions, so do your homework. Based on your sample, application and documented test results in your lab, select testing supplies with the highest potential for good results. Use best practices regarding sample and reagent preparation, dye volume, gelling time and temperature, destaining, storage and other critical tasks. Finally, remember your gloves!