What is a Calorimeter?
Laboratory calorimeters measure the enthalpy, or heat transfer under constant pressure, produced or absorbed by a chemical reaction or a change in a material’s physical state. For non-combustible materials, calorimeters are used to determine the sample’s heat capacity. Once the liquid or solid sample is combusted, the resulting temperature change, from the endothermic or exothermic reaction, is reported and used to determine the heat transfer resulting from the reaction.
How Does a Calorimeter Work?
Calorimeters include a decomposition vessel (also called a bomb), in which the solid or liquid sample is placed. The sample, housed within a crucible, is connected to an ignition wire by a cotton thread. The bomb vessel is then filled with an excess of oxygen to burn or combust the sample.
During combustion, the temperature of the decomposition vessel increases to near 1,000°C. To prevent disruptive external temperature or humidity influences, the entire system is encased in an insulated plenum, or jacket.
The heat produced during the reaction is captured and measured using different methods: adiabatic, dynamic, isoperibolic, static-jacketed, or double-dried. The results are displayed on a digital readout connected to a software program capable of saving methods and exporting data.
Calorimeters are utilized in many industries, including geothermal testing, injection molding, radionuclide characterization, and natural gas testing.
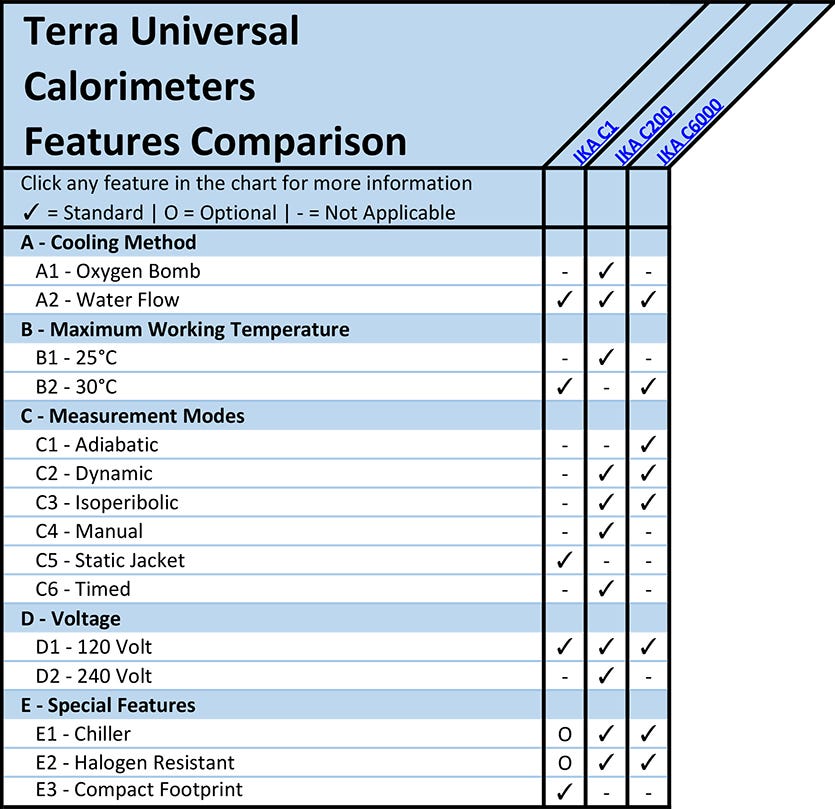
A - Calorimeter Cooling Method
(back to chart)
A1 - Oxygen Bomb Calorimeters
An oxygen bomb calorimeter or steel decomposition vessel containing the sample and high-pressure oxygen, is submerged in water while the mixture is ignited to ensure the energy produced by the reaction is contained.
A2 - Water Flow Calorimeters
Water-flow calorimeters feature a fluid reservoir, with automatic filling and draining, connected to an optional water heater or chiller for precise temperature control.
B - Maximum Calorimeter Working Temperature
(back to chart)
The maximum working temperature is the highest permissible ambient temperature under which the calorimeter can efficiently operate. As external conditions may impact the accuracy of results, all calorimeters must be operated under precisely controlled temperatures.
C - Measurement Modes
(back to chart)
C1 - Adiabatic Calorimeters
Adiabatic calorimeters ensure the temperature inside the insulated plenum, or jacket, remains equal to the temperature within the decomposition vessel during the test run. As an adiabatic system approaches perfect insulation, no correction calculations are required to account for external influences.
C2 - Dynamic Calorimeters
Dynamic calorimeters utilize the same measurement process as adiabatic systems, but the run time is shortened. Although the measurement results will fall within a standard deviation of the official standards, the accuracy and precision may be slightly lower. Dynamic calorimeters are commonly used in high-throughput labs processing a high volume of samples.
C3 - Isoperibolic Calorimeters
Isoperibolic calorimeters ensure the temperature within the jacket, or plenum, remains constant throughout the test run, resulting in lower heat flow. As an isoperibolic system does not trend toward perfect insulation, ambient conditions are tightly controlled to reduce temperature fluctuations. After the test, a correction factor is calculated and applied to the results.
C4 - Manual Calorimeters
IKA C200 calorimeters include a manual isoperibolic mode for employee training or higher education labs. The automated functions are switched off to allow for a trainer or professor to operate the system in a step-by-step fashion.
C5 - Static Jacket Calorimeters
In a static jacket calorimeter, the air-filled aluminum jacket is not temperature controlled. Although the air within the jacket plenum acts as a buffer to protect the calorimeter housing and sample vessel, a correction calculation must be applied to measured results, much like an isoperibolic system.
C6 - Timed Calorimeters
Double-dry (timed) calorimeters measure the temperature increase in the decomposition vessel rather than transferring the heat to the water inside the inner vessel, like isoperibolic and adiabatic systems. Without the heat transfer step, measurement times are reduced down to 3 minutes for standard samples. Double-dry calorimeters are commonly used by the waste management industry.
D - Calorimeter Voltage
(back to chart)
120-volt connections are suitable for standard laboratory power outlets in the United States.
240-volt connections, common in Mainland Europe, require less current (amperage) and smaller conductors than equipment designed to operate at 120-volt.
E - Special Features
(back to chart)
E1 - Calorimeter Chiller
Recirculating chillers improve reproducibility by regulating water conditions to provide stable starting temperatures for calorimetric measurements.
IKA RC2 basic recirculating chillers feature a 4 liter storage capacity and 400-Watt motor to cool water down to -20°C.
E2 - Halogen Resistant Calorimeter
For samples with high halogen content, IKA calorimeters include an optional, steel, halogen-resistant combustion chamber with catalytic-activated inner surface.
E3 - Compact Footprint Calorimeter
IKA’s C1 Calorimeter is the smallest commercially-available model on the market. Less thanthe one cubic foot in size, the C1 is optimal for crowded benchtop spaces in busy, shared-use labs.
Where Can I Buy Calorimeters Online?
Laboratory-Equipment.com is a specialty division of Terra Universal. For nearly 40 years, Terra Universal has served semiconductor, aerospace, life science, pharmaceutical, biotechnology, and medical device markets. Customers appreciate a worldwide network of reps, factory-direct support, and ready-to-ship items available from Terra's manufacturing and warehouse facilities in Fullerton, California.
Shop online to compare pricing, features, and selection for a wide variety of laboratory calorimeters, stirrers, shakers, and other equipment for applications including general laboratory, PCR, DNA/RNA techniques, ELISA, protein analysis, and cell culture.



